Production
Development and Production of Disinfectants

BOZON has been carrying out its production and research activities since 2008, when the first unique formulations were developed and the first batch of professional disinfectants under its own brand was produced. Today, our production means modern, high-tech equipment of the world class, qualified staff with extensive experience, innovative technologies, and strict observance of the required conditions of production, storage, and transportation of finished products.
The bases for the safety and high quality of our products are as follows:
- In-house chemical and microbiological laboratories using physical and chemical methods of analysis (such as photometry, gas-liquid chromatography, pH measurement, and various titration methods).
- Production capacities in the Moscow Region based in OAO PO TOS* and AO Voskresensky NIUiF**.
- Environmentally friendly raw materials, substances, and components from leading domestic and global suppliers.
- Strict control at each stage of production and stable effect of each batch of products.
- Compliance with the requirements of Russian and global production standards.
Disinfectants, antiseptics, and hygiene products are of particular importance in the prevention of infections associated with the medical care, as well as the disinfection and hygiene in various fields. Our products are ready-made solutions and concentrates with high antibacterial and antiviral activity and can be used to treat various surfaces, tools, equipment, as well as human hands and skin. Besides, all the products have low toxicity, do not contain allergens, are safe for humans, easy to use, do not damage items subject to treatment, and have a long shelf life.
The requirements of sanitary-epidemiological rules and regulations, as well as sectoral and legislative norms form the basis for the development and manufacturing of our products. Experienced chemists and virologists, epidemiologists, and sanitary doctors are among the Company's employees and consultants. All the products developed by BOZON undergo the mandatory state registration procedure, have declarations of conformity and product instructions approved in accordance with the established procedure.
* OAO PO TOS — Open Joint-Stock Company “Fine Organic Synthesis Production Association”.
**AO Voskresensky NIUiF — Joint-Stock Company “Voskresensky Research and Development Institute for Fertilizers and Phosphoric Acid”.
Production of Chevron Special-Purpose Brushes
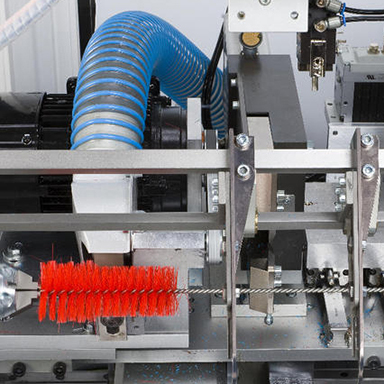
Endoscopic procedures are gaining increasing popularity in the contemporary medicine, and medical instruments are becoming more complex and technological from year to year. Taking into account the importance of the instrument cleaning in medical institutions, our Company started the production of special brushes under the Chevron brand designed for mechanical cleaning of endoscopes and other hollow instruments before the subsequent treatment.
BOZON experts developed a diverse range of disposable and reusable brushes designed for comprehensive cleaning of endoscopes and other hollow instruments from various manufacturers. Manual mechanical cleaning is the most important stage of instrument treatment. Further sterilization of an instrument is of no effect unless it has been cleaned. Proper cleaning prolongs the life of the instrument and increases the reliability of its work.
Kits of disposable Chevron brushes are designed for cleaning external and internal surfaces of flexible and rigid endoscopes and instruments for them, as well as for cleaning complex surgical instruments with cavities and locking parts. The brushes in the kits have been selected in the most convenient way in the optimal range of sizes, styles, and materials of bristles to cover the maximum possible number of instrument types to be cleaned. Reusable Chevron brushes are available with various diameters, lengths, and hardness and are designed to clean internal and external surfaces, as well as to clean jaws and sharp, piercing surfaces of various medical instruments. Reusable brushes are resistant to disinfection, pre-sterilization cleaning, and sterilization in accordance with the respective guidelines.
Modern high-quality materials, innovative technologies, and compliance with production standards guarantee the high quality of the products manufactured. All the brushes we produce have passed the state registration procedure and have the required registration certificates and product instructions.
